The neutral atom chlorine Z17 for instance has 17 electrons. You should take a look at section 363 and look closely at the first 20 elements.

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry
The main properties that can be compared is the melting.

. Assume G is lighter than RnThink about the chemical elements made of atoms with an electron configuration that matches each pattern. Starting with simple elements the first three rows of the periodic table called Periods 1 2 and 3 correspond to the n1 n2 and n3 levels. Looking at the periodic table you can see that Oxygen has 8 electrons.
Electron shell configurations of the first 18 elements. Electron Configuration Pattern - 17 images - iron orbital and bonding info chemistry notes on atomic structure electron electron waves. ANALYZING DATA Patterns in Electron Configurations As described by the quantum mechanical model of the atom every electron has a precise amount of energy and occupies a specific atomic orbital.
Too often students remember for a short time certain trends and properties associated with groups and periods of elements yet come away from a study of the periodic table without really understanding or appreciating how much information the table. Electron Configurations Filling Pattern - 19 images - electron configurations of the elements 1 5 atomic orbitals chemistry libretexts activity patterns in electron configuration names patterns in electron configuration worksheet answer key. Explaining Patterns in the Periodic Table Identifying family and period properties within the periodic table.
Compare the electron configuration of an element and its position on the periodic table. Mendeleev made an early periodic table. The electron shell configurations of the first 18 elements in the periodic table.
The study of organic chemistry must at some point extend to the molecular level for the physical. Nitrogen can lose all 5 electrons resulting in a 5 charge. Compare the electron configuration of an element and its position on the periodic table.
Step 2 Thus beryllium has an He2 s2 electron configuration. They are in column 15 on the periodic table. They have an ionic charge of 4- or 4 respectively.
Describe Patterns in Electron Configuration in Periods Structure Bonding. The periodic table shown here is severely truncated. This periodic table contains each elements atomic number atomic mass symbol name and electron configuration.
Up to 24 cash back The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right. Electronic configuration is also referred to as electron configuration. They are in column 14 of the periodic table.
So Oxygens electron configuration would be O 1s22s22p4. Based on the order of fill above these 8 electrons would fill in the following order 1s 2s and then 2p. As you complete the activity keep the following in mind.
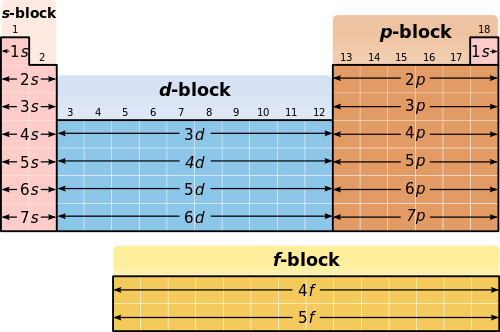
Electron Configurations in the Periodic Table. The corresponding energy levels n are listed in green numbers to the left. In what block or blocks of the Periodic Table.
See Figure 1 Figure 1. In this activity you will identify these patterns. This notation uses the symbol of the previous rows noble gas in brackets to represent the part of the electron configuration that is identical to that noble gass electron.
3H - Electron Configuration and the Periodic Table. The key way to understand the basics of the electrons is the number of shells and the number of electrons they can hold. In order to write electron configurations in short form it is handy to look at a periodic table shown previously and use the pattern of the s p d and f blocks to determine the electrons that.
What is the electronic configuration of chlorine. An electron configuration is the way that the electrons of an atom are arranged in the atomic orbitals. The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element.
Patterns in Electron Configuration One of the many patterns contained in the periodic table is that of electron configuration. Step 1 The group 2 elements are in the s block of the periodic table and as group 2 elements they all have two valence. Beginning with hydrogen and continuing across the periods of the periodic table we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the elements.
Electronic configurations model how electrons are arranged in atoms. It also has rows called periods and columns called groups. To determine the electron configuration for any particular atom we can build the structures in the order of atomic numbers.
Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom. This table is set up in order of increasing atomic number. N and P have 5 valence level electrons so they will likely gain 3 electrons resulting in a 3- charge.
The first shell can hold 2 electrons the second shell can hold 8 electrons and the 3rd shell can hold 18. For example all the electron configuration of all elements in group 2 can be expressed in the form X ns² where X is the configuration of the noble gas from the preceding period and n is the principal quantum number. Some patterns of electron configuration are listed below.
The electron configurations are written in the noble gas notation. This plays a very important role in the structure of the periodic table and some of the trends and patterns of reactivity. Chlorine has an electron configuration of 1s2 2s2 2p6 3s2 3p5.
This structure gives the ground state electron configuration for any atom. The pattern of elements in the periodic table reflects the progressive filling of electronic orbitals. Scientists have identified three rules that explain the most stable electron.
You should take a look at and look closely at the first 20 elements. There are exceptions to this. So it makes sense that the structure of the periodic table reflects periodic trends in the electron configuration of elements.
There are 4 quantum numbers associated with any electron that describe its existence in space we will concern ourselves with two one of energy. If you consider the electronic configuration of an atom of each element in the Periodic Table you will see a number of patterns which are referred to as periodic trends or just trends. In each case G stands for a noble-gas core and n m or o stand for integer numbers like 1 2 3and so on.
There is a pattern between the electron configuration for the elements and their positions on the periodic table. Therefore its ground state electronic. The next element down magnesium is expected to have.
Filling of the s subshells. There is a pattern between the electron configuration for the elements and their positions on the periodic table. How many electron configurations does a chlorine atom have.
Elements belonging in Group IA eg - H Li Na K all have electron configuration ending in ns 1. The two columns on the leftthe alkali metals and alkaline earthsshow the addition of 1 and 2 electrons into s type subshells. Chlorine is found in the group 17 the halogens on the periodic table.
In the modern periodic table elements are in order of atomic number in periods and groups.

Electron Configurations The Cavalcade O Chemistry

Writing Electron Configurations Using Only The Periodic Table Youtube

Dublin Schools Lesson Electron Configurations Using The Periodic Table
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Electron Configuration And The Modern Periodic Table Examples Pedia

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com
How To Use The Periodic Table To Write Electron Configurations For Each Element Quora

0 komentar
Posting Komentar